Supercritical Fluid Application in the Oil and Gas Industry: A Comprehensive Review
Abstract
:1. Introduction
- (1)
- possessing the properties of gases at high pressures (low viscosity, high diffusion coefficient) and liquids (high solubility),
- (2)
- high mass transfer rate due to low viscosity and high diffusion coefficient; combining negligible interfacial tension with low viscosity and high diffusion coefficient, allowing supercritical fluids to penetrate porous media more easily, as compared with ordinary liquids,
- (3)
- the high sensitivity of the solvent capacity of SCF to changes in pressure or temperature, and
- (4)
- easy separation of SCF and dissolved substances during pressure relief.
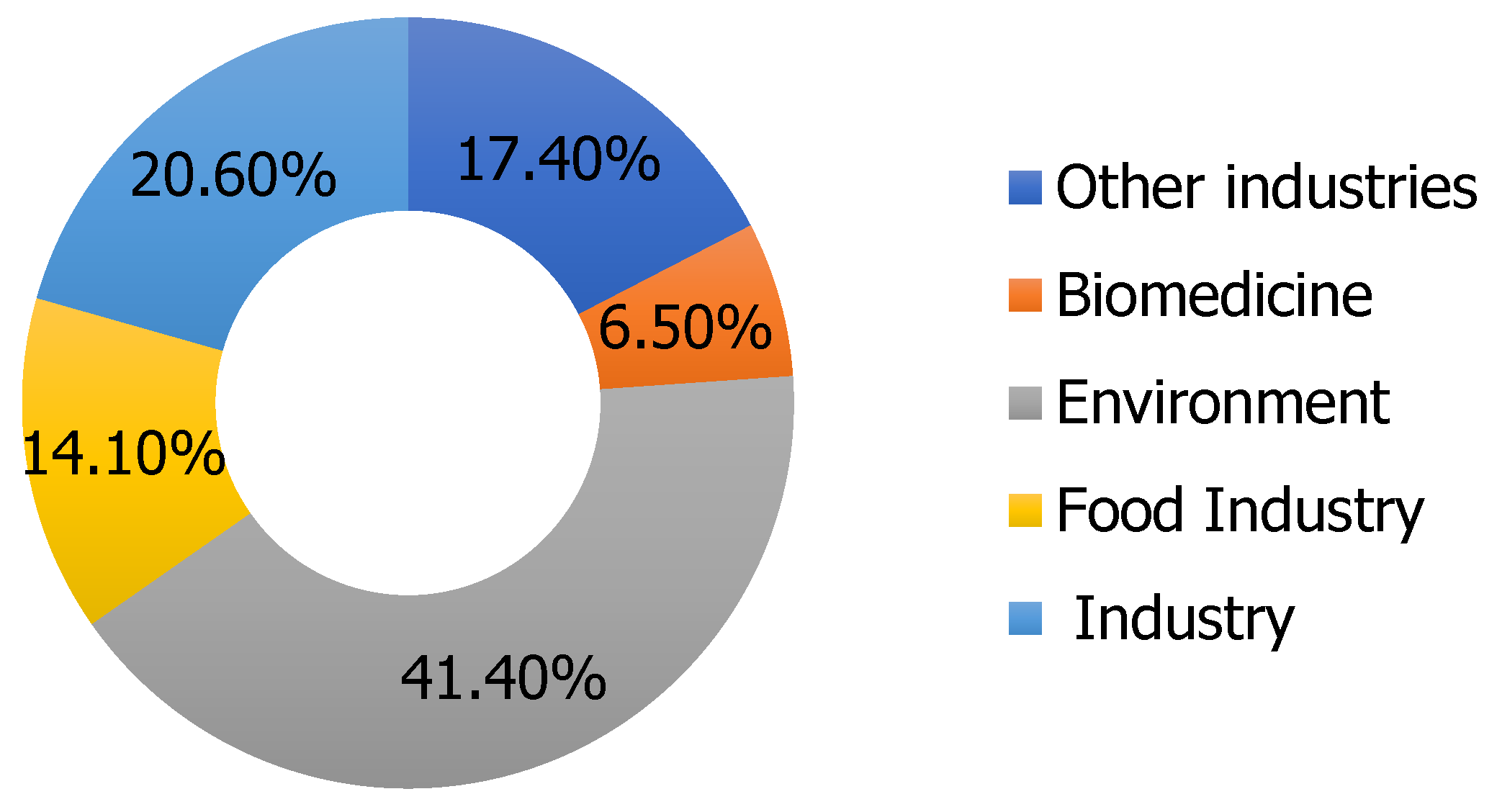
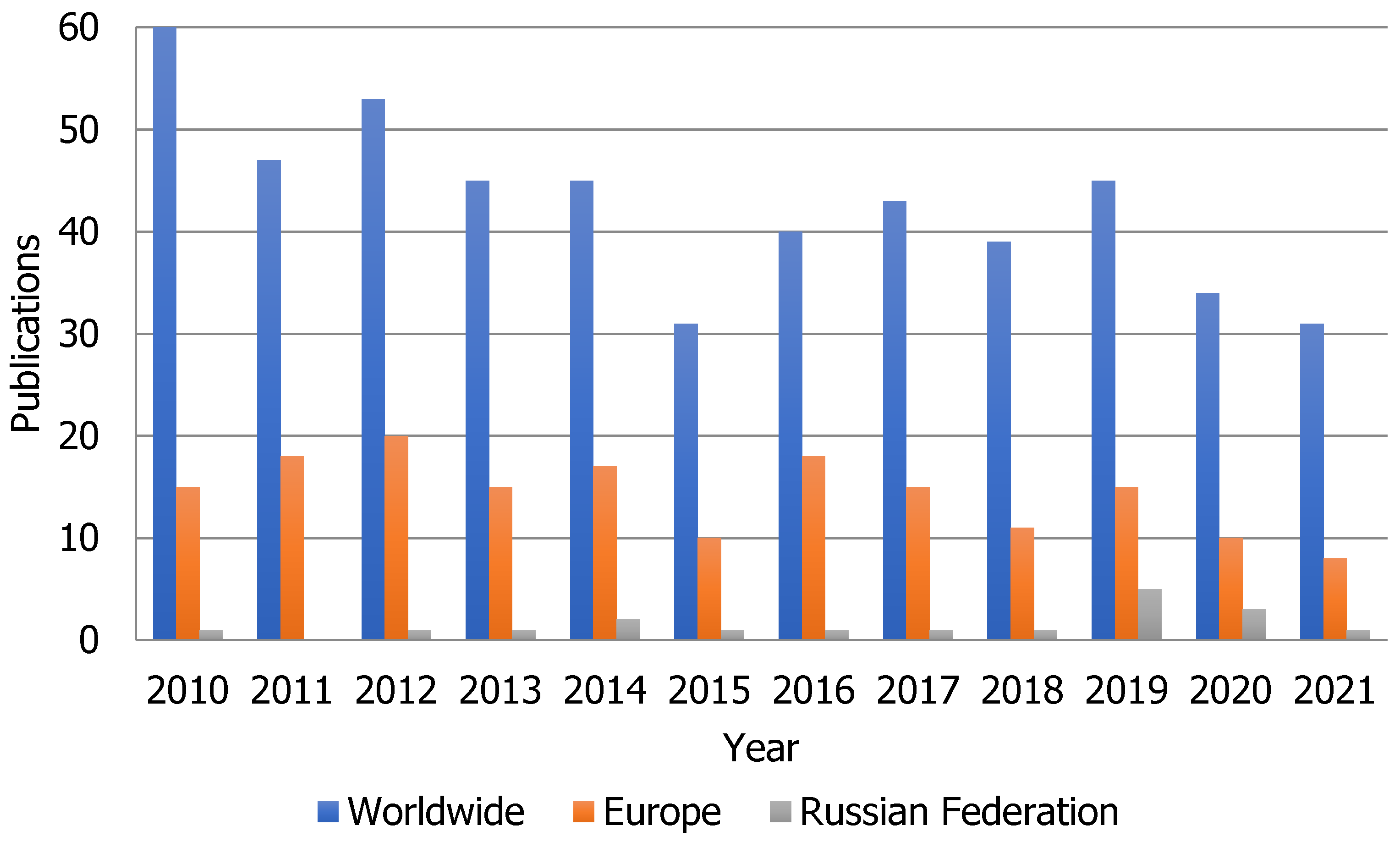
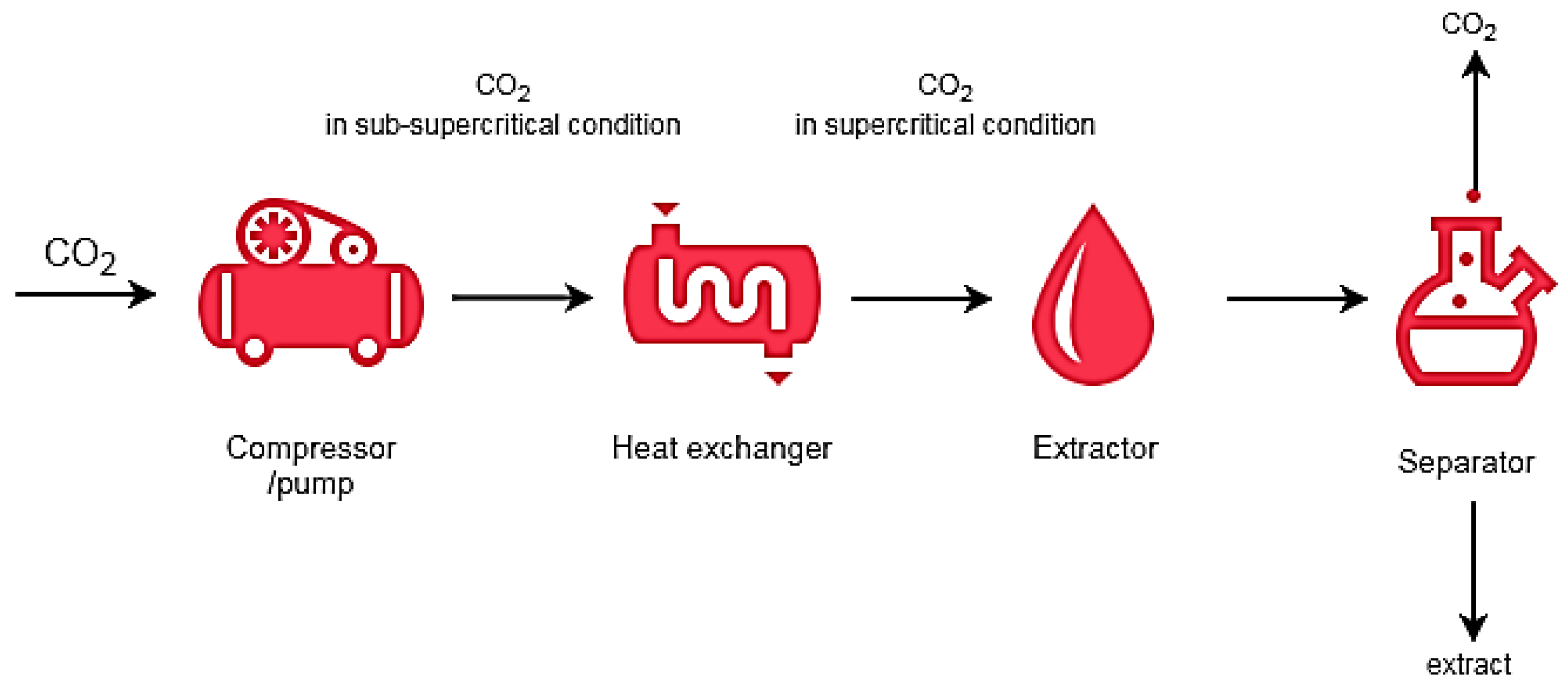
2. Supercritical Extraction
3. Increasing Oil Recovery by Carbon Dioxide Pumped into the Reservoir
| Ref. | Type of Fluid | Condition of Fluid | Pressure, MPa | Temperature, °C | Type of Displacement | Type of Core | Results |
|---|---|---|---|---|---|---|---|
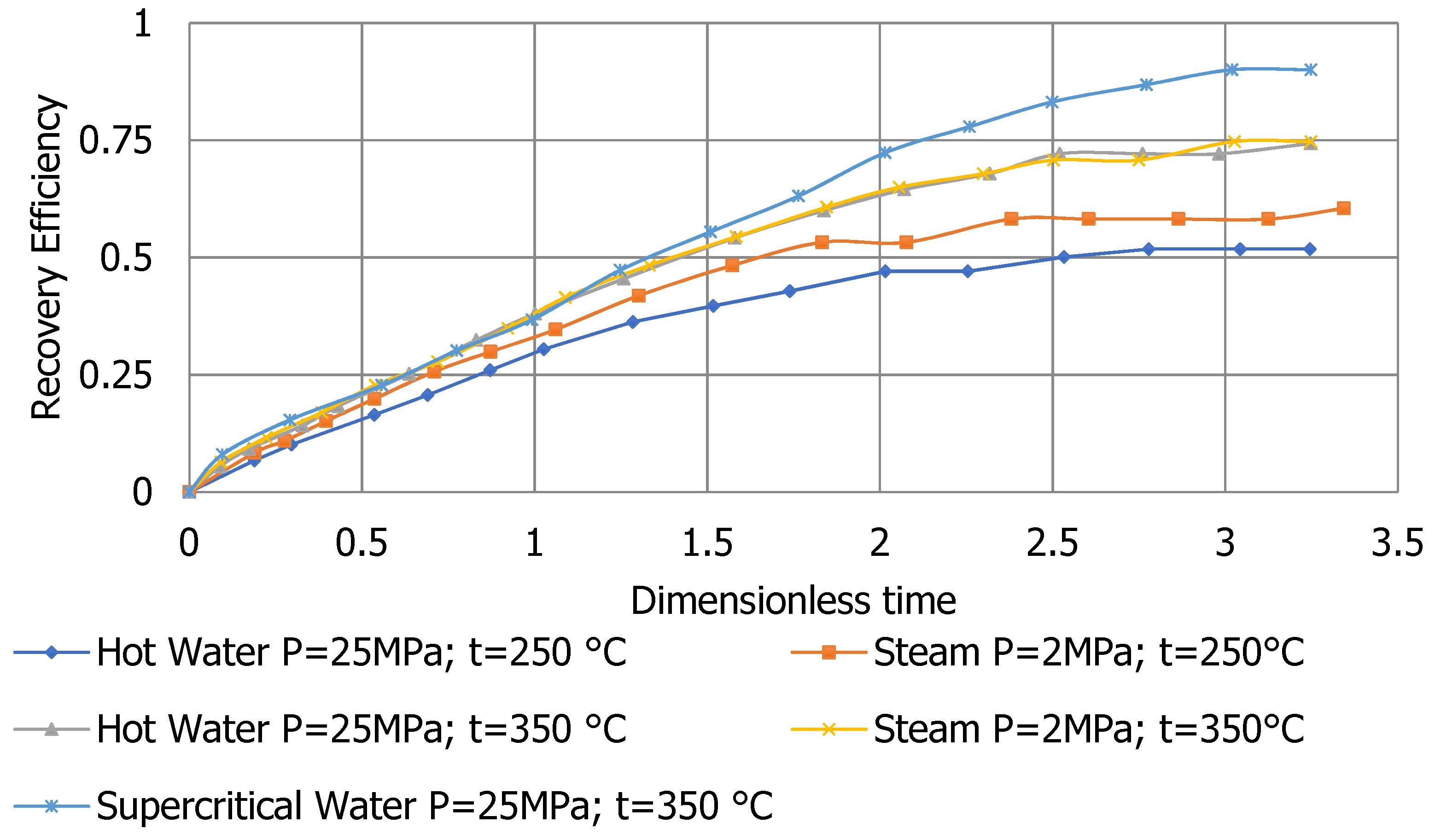
| Qiuyang Zhao et al., 2020 [18] | H2O | SCF | 25 | 400 | Miscible | Sand | Improved oil recovery by 17% compared to steam |
| Phong Nguyen et al., 2018 [64] | CO2 | SCF | about 5 to 8 | 50 | Miscible | Microfluidic chips, the connected fracture network | Oil recovery 90% |
| CO2 | SCF | about 5 to 8 | 50 | Miscible | Microfluidic chips, the dead-end fracture network | Oil recovery 60% | |
| N2 | Gas | about 5 to 8 | 50 | Miscible | Microfluidic chips, the connected fracture network | Oil recovery 40% | |
| N2 | Gas | about 5 to 8 | 50 | Miscible | Microfluidic chips, the dead-end fracture network | Oil recovery 25% | |
| H2O | Liquid | about 5 to 8 | 50 | Immiscible | Microfluidic chips, the connected fracture network | Not effective | |
| H2O | Liquid | about 5 to 8 | 50 | Immiscible | Microfluidic chips, the dead-end fracture network | Not effective | |
| Duraid Al-Bayati et al., 2017 [77] | CO2 | SCF | 17.2 | 69.85 | Miscible | Sand | Oil recovery 93.5% |
| CO2 | SCF | 9.6 | 69.85 | Immiscible | Oil recovery 76% | ||
| Sai Wang et al., 2019 [78] | CO2 | SCF | 17.23 | 40 | Immiscible | Core from the Bakken formation | Oil recovery ~ 8.8% |
| CO2 | SCF | 19.3 | 40 | Miscible | Oil recovery ~12% | ||
| CO2 | SCF | 20.68 | 40 | Miscible | Oil recovery ~12.3% | ||
| CO2 | SCF | 22.06 | 40 | Miscible | Oil recovery ~33% | ||
| Abdulrazag Yusef Zekri; Reyadh A. Almehaideb; Shedid A. Shedid 2006 [80] | CO2 | SCF | 11.03 | 52.78 | Immiscible | 0.16 mD permeability, carbonate rocks | Oil recovery ~45% |
| CO2 | SCF | 11.03 | 52.78 | Immiscible | 11.99 mD permeability, carbonate rocks | Oil recovery ~95% | |
| F. Irawan, S. Irawan & M. Awang 2012 [82] | CO2 | Liquid | 10.34 | 20 | No information | Berea sandstone | Oil recovery 67.7% |
| CO2 | Liquid | 10.34 | 11.67 | Oil recovery 69.1% | |||
| CO2 | Liquid | 10.34 | 5 | Oil recovery 72.6% | |||
| Manoj Kumar Valluri et al., 2020 [83] | CO2 | Gas | <9.14 | 32.78 | Immiscible | 12.56 mD permeability, Copper Ridge Dolomite | Average oil recovery 16% |
| CO2 | SCF | >9.14 | 32.78 | Miscible | 12.56 mD permeability, Copper Ridge Dolomite | Average oil recovery 26% | |
| H2O | Liquid | 9.14 | 32.78 | - | 12.56 mD permeability, Copper Ridge Dolomite | Average oil recovery 30% | |
| CO2 | SCF | <9.14 | 32.78 | Immiscible | 2,74 mD permeability, Clinton Sandstone | Average oil recovery 29% | |
| CO2 | SCF | >9.14 | 32.78 | Miscibility | 2.74 mD permeability, Clinton Sandstone | Average oil recovery 26% | |
| H2O | Liquid | 9.14 | 32.78 | - | 2.74 mD permeability, Clinton Sandstone | Average oil recovery 20% | |
| Mehdi Fahandezhsaadi et al., 2019 [84] | N2 | Gas | 6.89 | 70 | Immiscible | Carbonate rocks | Oil recovery ~40% |
4. Using SCF for Processing and Disposal of Oil Sludge
5. Using SCF for Hydraulic Fracturing
6. Using SCF for Cleaning Heavy Oil Deposits
7. The Effect of SCF on the Equipment Corrosion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Author of the Methodology | Calculation Formula |
|---|---|---|
| 1 | Poynting equation [54] | where Pv is the saturation pressure, Pa; is the saturation pressure of the externally compressed condensed medium, Pa; VL is the specific volume of the condensed medium, m3/kg; μ is the molar volume of the condensed medium, m3/mol; R is the gas constant, J/(kg·K); T is the absolute temperature, K; P is the external pressure, Pa. |
| 2 | Bilalov-Gumerov equation [51,54] | where y is the solubility of the studied substance in the fluid, g/L; is the chemical potential of supercritical fluid at process temperatures, J/mol; is supercritical fluid density, kg/m3; is supercritical temperature, K; φ is volume fraction of solvent, %; are molar entropies, J/(mol·K); is the scale flow of the dissolved component through the unit thickness of the fluid layer when the entropy changes at the interface ; R is the universal gas constant, J/(kg·K); i is the association number. |
| 3 | Chrastil equation [55] | where S is the solubility of the solute in dense gas, expressed in g/L; is the density of the solvent, m3/kg; k is the association number; T is the temperature, K; α is the reaction heat; b is the ratio between the molecular masses of the solute and the solvent where ΔH is the total reaction heat, J/mol; R is the gas constant, J/(kg·K); MA and MB are the molecular weights of the solute and of the gas, correspondingly, u; q is a constant. |
| 4 | Del Valle-Aguilera equation [56] | where c is an empirical modification introduced by Del Valle-Aguilera to account for the evaporation heat of the solute. |
| 5 | Adachi-Lu equation [57] | where k, a, b, c, and d are coefficients adjusted by fitting to experimental data. |
| 6 | A modified model of the mass action law for the correlation of solubility of solid and liquid substances in SCF [58] | is the density of pure SCF, kg/m3; k is the snumber of SCF associations; m is the concentration of co-solvent (mole fraction ) in a binary mixture consisting of SCF and co-solvent; γ is the number of associations for co-solvent. |
| 7 | Solubility equation of solid active ingredients in sc-CO2 with and without co-solvents [59] | The new q-Chrastil’s equation uses the definition of the q-exponential function expressed as follows [60]: Applying the q-exponential function, it is possible to express q-Chrastil’s equation as is the density of pure SCF, kg/m3; k is the number of SCF associations; m is the concentration of co-solvent (mole fraction) in a binary mixture consisting of SCF and co-solvent; γ is the number of associations for co-solvent; the parameter is related to the solvation energy. |
References
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- Ortiz, F.J.G.; Kruse, A. The use of process simulation in supercritical fluids applications. React. Chem. Eng. 2020, 5, 424–451. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Aminabhavi, T.M. A Review on Extraction and Identification of Crude Oil and Related Products Using Supercritical Fluid Technology. Energy Fuels 2000, 14, 464–475. [Google Scholar] [CrossRef]
- Fukushima, Y. Application of Supercritical Fluids. Rev. Toyota CRDL 1999, 35, 1–9. [Google Scholar]
- Zalepugin, D.Y.; Tilkunova, N.A.; Chernyshova, I.V.; Polyakov, V.S. Development of Technologies based on the use of Supercritical Fluids. Supercrit. Fluids Theory Pract. 2006, 1, 27–51. [Google Scholar]
- Said-Galiev, E.E.; Nikitin, L.N.; Khokhlov, A.R. Synthesis of polyimides in supercritical carbon dioxide. In Proceedings of the International Scientific and Practical Conference “Supercritical Fluid Technologies: Innovative Potential of Russia”, Rostov-on-Don, Russia, 29 June–1 July 2004; pp. 85–87. [Google Scholar]
- Zougagh, M.; Valcárcel, M.; Ríos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Kamal, G.; Sumit, A.; Anil, K. Industrial applications of supercritical fluid extraction: A review. Int. J. Chem. Stud. 2017, 5, 336–340. [Google Scholar]
- Trukhina, O.S.; Sintsov, I.A. Experience of using carbon dioxide to improve oil recovery of reservoirs. Uspekhi Sovrem. Estestvozn. 2016, 3, 205–209. Available online: https://natural-sciences.ru/ru/article/view?id=35849 (accessed on 29 November 2021).
- Roopa, I.; Dawe, R.A. A Laboratory Study of Recovery with Carbon Dioxide around Critical Conditions of Trinidad’s Heavy Oil and Tar Sands. Pet. Sci. Technol. 2010, 28, 1544–1554. [Google Scholar] [CrossRef]
- Rezk, M.; Foroozesh, J. Effect of CO2 mass transfer on rate of oil properties changes: Application to CO2-EOR projects. J. Pet. Sci. Eng. 2019, 180, 298–309. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Yu, W.; Wang, H.; Chen, Z.; Sun, H.; Zhang, S. Thermodynamic Behavior of Liquid-Supercritical CO2 Fracturing in Shale. Presented at the SPE/AAPG/SEG Unconventional Resources Technology Conference, Austin, TX, USA, 17 July 2017. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Wang, B.; Shen, Z.; Zheng, Y.; Jia, Z.; Yan, W. Digital quantification of fracture in full-scale rock using micro-CT images: A fracturing experiment with N2 and CO2. J. Pet. Sci. Eng. 2020, 196, 107682. [Google Scholar] [CrossRef]
- Chen, Y.; Pope, T.L.; Lee, J.C. Novel CO2–Emulsified Viscoelastic Surfactant Fracturing Fluid System. In Proceedings of the SPE European Formation Damage Conference, Sheveningen, The Netherlands, 25–27 May 2005. [Google Scholar] [CrossRef]
- Ishida, T.; Desaki, S.; Yamashita, H.; Inui, S.; Naoi, M.; Fujii, H.; Katayama, T. Injection of Supercritical Carbon Dioxide into Granitic Rock and its Acoustic Emission Monitoring. Procedia Eng. 2017, 191, 476–482. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, L.; Wang, Y.; Jin, H.; Chen, L.; Huang, Z. Enhanced Oil Recovery and in Situ Upgrading of Heavy Oil by Supercritical Water Injection. Energy Fuels 2020, 34, 360–367. [Google Scholar] [CrossRef]
- Khat, V.E. Fluidodynamic Factor in Tectonics and Oil and Gas Content of Sedimentary Basins; Nauka: Moscow, Russia, 1989; 239p. [Google Scholar]
- Wayles, S. Phase Equilibria in Chemical Technology: Translated from English; Wayles, S., Translator; Beskov, V.S., Ed.; Mir: Moscow, Russia, 1989; Chapter 1; 304p. [Google Scholar]
- Johnson, O.A.; Affam, A.C. Petroleum sludge treatment and disposal: A review. Environ. Eng. Res. 2019, 24, 191–201. [Google Scholar] [CrossRef]
- White, M.T.; Bianchi, G.; Chai, L.; Tassou, S.A.; Sayma, A.I. Review of supercritical CO2 technologies and systems for power generation. Appl. Therm. Eng. 2021, 185, 116447. [Google Scholar] [CrossRef]
- Hauthal, W.H. Advances with supercritical fluids [review]. Chemosphere 2001, 43, 123–135. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P. Supercritical Fluid Technology: A Review. J. Adv. Pharm. Sci. Technol. 2013, 1, 13–36. [Google Scholar] [CrossRef] [Green Version]
- Rozzi, N.L.; Singh, R.K. Supercritical Fluids and the Food Industry. Compr. Rev. Food Sci. Food Saf. 2006, 1, 33–44. [Google Scholar] [CrossRef]
- Yin, J.; Zheng, Q.; Peng, Z.; Zhang, X. Review of supercritical CO 2 power cycles integrated with CSP. Int. J. Energy Res. 2019, 44, 1337–1369. [Google Scholar] [CrossRef]
- Licence, P.; Ke, J.; Sokolova, M.; Ross, S.K.; Poliakoff, M. Chemical reactions in supercritical carbon dioxide: From laboratory to commercial plant. Green Chem. 2003, 5, 99–104. [Google Scholar] [CrossRef]
- Wai, C.M.; Hunt, F.; Ji, M.; Chen, X. Chemical Reactions in Supercritical Carbon Dioxide. J. Chem. Educ. 1998, 75, 12–1641. [Google Scholar] [CrossRef]
- Amandi, R.; Hyde, J.R.; Ross, S.K.; Lotz, T.J.; Poliakoff, M. Continuous reactions in supercritical fluids; a cleaner, more selective synthesis of thymol in supercritical CO2. Green Chem. 2005, 7, 288–293. [Google Scholar] [CrossRef]
- Martins, E.; Aranda, D.; Pessoa, F.; Zotin, J. Hydrogenation of diesel aromatic compounds in supercritical solvent environment. Braz. J. Chem. Eng. 2000, 17, 361–366. [Google Scholar] [CrossRef]
- Kwek, W.; Khan, M.K.; Sarkar, B.; Kim, J. Supercritical methanol as an effective medium for producing asphaltenes-free light fraction oil from vacuum residue. J. Supercrit. Fluids 2017, 133, 184–194. [Google Scholar] [CrossRef]
- Webb, P.; Sellin, M.F.; Kunene, T.E.; Williamson, S.; Slawin, A.; Cole-Hamilton, D.J. Continuous Flow Hydroformylation of Alkenes in Supercritical Fluid−Ionic Liquid Biphasic Systems. J. Am. Chem. Soc. 2003, 125, 15577–15588. [Google Scholar] [CrossRef]
- Burgener, M.; Ferri, D.; Grunwaldt, J.-D.; Mallat, A.T.; Baiker, A. Supercritical Carbon Dioxide: An Inert Solvent for Catalytic Hydrogenation? J. Phys. Chem. B 2005, 109, 16794–16800. [Google Scholar] [CrossRef]
- More, S.R.; Yadav, G.D. Effect of Supercritical CO2 as Reaction Medium for Selective Hydrogenation of Acetophenone to 1-Phenylethanol. ACS Omega 2018, 3, 7124–7132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, J.; Han, B.; George, M.W.; Yan, H.; Poliakoff, M. How Does the Critical Point Change during a Chemical Reaction in Supercritical Fluids? A Study of the Hydroformylation of Propene in Supercritical CO2. J. Am. Chem. Soc. 2001, 123, 3661–3670. [Google Scholar] [CrossRef]
- Gordon, C.M.; Leitner, W. Catalyst Separation and Recycling; Springer: Dordrecht, The Netherlands, 2006; p. 215. [Google Scholar]
- Koch, T.J.; Desset, S.L.; Leitner, W. Catalytic supercritical fluid extraction: Selective hydroformylation of olefin mixtures using scCO2 solubility for differentiation. Green Chem. 2010, 12, 1719–1721. [Google Scholar] [CrossRef]
- Rathke, J.W.; Klingler, R.J.; Krause, T.R. Propylene hydroformylation in supercritical carbon dioxide. Organometallics 1991, 10, 1350–1355. [Google Scholar] [CrossRef]
- Fürstner, A.; Ackermann, L.; Beck, K.; Hori, H.; Koch, D.; Langemann, K.; Liebl, M.; Six, C.; Leitner, W. Olefin Metathesis in Supercritical Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 9000–9006. [Google Scholar] [CrossRef]
- Astruc, D. The metathesis reactions: From a historical perspective to recent developments. New J. Chem. 2005, 29, 42–56. [Google Scholar] [CrossRef]
- Cooper, A.I. Polymer synthesis and processing using supercritical carbon dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Yeo, S.-D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Boyère, C.; Jérôme, C.; Debuigne, A. Input of supercritical carbon dioxide to polymer synthesis: An overview. Eur. Polym. J. 2014, 61, 45–63. [Google Scholar] [CrossRef]
- Beckman, E.J. Oxidation Reactions in CO2: Academic Exercise or Future Green Processes? Environ. Sci. Technol. 2003, 37, 5289–5296. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castillo, Z.K.; Aki, S.N.V.K.; Stadtherr, M.A.; Brennecke, J.F. Enhanced Solubility of Oxygen and Carbon Monoxide in CO2-Expanded Liquids. Ind. Eng. Chem. Res. 2006, 45, 5351–5360. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Gonzalez, M.; Enriquez, J.; Zhao, Q. Selective Oxidation in Supercritical Carbon Dioxide Using Clean Oxidants. Ind. Eng. Chem. Res. 2000, 39, 4858–4864. [Google Scholar] [CrossRef]
- Dunn, J.B.; Urquhart, D.I.; Savage, P.E. Terephthalic Acid Synthesis in Supercritical Water. Adv. Synth. Catal. 2002, 344, 385–392. [Google Scholar] [CrossRef]
- Kim, D.S.; Shin, Y.; Lee, Y.-W. Synthesis of terephthalic acid by catalytic partial oxidation of p-xylene in supercritical carbon dioxide. Chem. Eng. Commun. 2014, 202, 78–84. [Google Scholar] [CrossRef]
- Grumett, P. Precious Metal Recovery from Spent Catalysts. Platinum Met. Rev. 2003, 47, 163–166. Available online: https://www.technology.matthey.com/article/47/4/163-166/ (accessed on 29 November 2021).
- URACA Company. High-Pressure Pumps. Available online: https://www.uraca.com/ru/sistemy-i-izdelija/nasosy/ (accessed on 29 November 2021).
- Bilalov, T.L.; Gumerov, F.M. Calculation of the solubility of aromatic hydrocarbons in supercritical media based on the entropy method of similarity theory. Theor. Found. Chem. Technol. 2019, 4, 387–401. [Google Scholar]
- Carmela, S.R. Experiments and Modeling of Supercritical CO2 Extraction of Lipids from Microalgae. Master’s Thesis, University of Padua, Padua, Italy, 2014; 164p. [Google Scholar]
- Rój., E. Supercritical CO2 Extraction and Its Applications; Polish Foundations of the Opportunities Industralization Centers “OIC Poland”: Lublin, Poland, 2014. [Google Scholar]
- Gumerov, F.M.; Sabirzyanov, A.N.; Gumerova, G.I. Sub-and Supercritical Fluids in Polymer Processing Processes; AN RT “FENG”: Kazan, Russia, 2000; 328p. [Google Scholar]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Adachi, Y.; Lu, B.C.-Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilibria 1983, 14, 147–156. [Google Scholar] [CrossRef]
- González, J.C.; Vieytes, M.R.; Botana, A.M.; Vieites, J.M.; Botana, L.M. Modified mass action law-based model to correlate the solubility of solids and liquids in entrained supercritical carbon dioxide. J. Chromatogr. A 2001, 910, 119–125. [Google Scholar] [CrossRef]
- Tabernero, A.; de Melo, S.V.; Mammucari, R.; del Valle, E.M.; Foster, N. Modelling solubility of solid active principle ingredients in sc-CO2 with and without cosolvents: A comparative assessment of semiempirical models based on Chrastil’s equation and its modifications. J. Supercrit. Fluids 2014, 93, 91–102. [Google Scholar] [CrossRef]
- Borges, E.P. On aq-generalization of circular and hyperbolic functions. J. Phys. A Math. Gen. 1998, 31, 5281–5288. [Google Scholar] [CrossRef]
- Nbs Technical Note 1070; Ely, J.F.; Baker, J.K. A Review of Supercritical Fluid Extraction; National Bureau of Standards Technical Note: Washington, DC, USA, 1983; p. 77.
- Zuknik, M.H.; Norulaini, N.N.; Dalila, W.W.N.; Ali, N.R.; Omar, A.M. Solubility of virgin coconut oil in supercritical carbon dioxide. J. Food Eng. 2016, 168, 240–244. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Turczyn, A. Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide. Molecules 2019, 24, 4174. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.; Carey, J.W.; Viswanathan, H.S.; Porter, M. Effectiveness of supercritical-CO2 and N2 huff-and-puff methods of enhanced oil recovery in shale fracture networks using microfluidic experiments. Appl. Energy 2018, 230, 160–174. [Google Scholar] [CrossRef]
- Yellig, W.F.; Metcalfe, R.S. Determination and Prediction of CO2 Minimum Miscibility Pressures (includes associated paper 8876). J. Pet. Technol. 1980, 32, 160–168. [Google Scholar] [CrossRef]
- Christiansen, R.L.; Haines, H.K. Rapid Measurement of Minimum Miscibility Pressure with the Rising-Bubble Apparatus. SPE Reserv. Eng. 1987, 2, 523–527. [Google Scholar] [CrossRef]
- Rao, D.N. A new technique of vanishing interfacial tension for miscibility determination. Fluid Phase Equilibria 1997, 139, 311–324. [Google Scholar] [CrossRef]
- Yuan, H.; Johns, R.; Egwuenu, A.; Dindoruk, B. Improved MMP Correlations for CO2 Floods Using Analytical Gas Flooding Theory. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004; pp. 418–425. [Google Scholar] [CrossRef]
- Glaso, O. Generalized Minimum Miscibility Pressure Correlation (includes associated papers 15845 and 16287 ). Soc. Pet. Eng. J. 1985, 25, 927–934. [Google Scholar] [CrossRef]
- Hassan, A.; Elkatatny, S.; Abdulraheem, A. Intelligent Prediction of Minimum Miscibility Pressure (MMP) During CO2 Flooding Using Artificial Intelligence Techniques. Sustainability 2019, 11, 7020. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Luo, P. Experimental and simulation determination of minimum miscibility pressure for a Bakken tight oil and different injection gases. Petroleum 2017, 3, 79–86. [Google Scholar] [CrossRef]
- Knapik, E.; Chruszcz-Lipska, K. Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland. Energies 2020, 13, 6456. [Google Scholar] [CrossRef]
- Brown, K.; Whittaker, S.; Wilson, M.; Srisang, W.; Smithson, H.; Tontiwachwuthikul, P. The history and development of the IEA GHG Weyburn-Midale CO2 Monitoring and Storage Project in Saskatchewan, Canada (the world largest CO2 for EOR and CCS program). Petroleum 2017, 3, 3–9. [Google Scholar] [CrossRef]
- Jones, A.C.; Lawson, A.J. Carbon Capture and Sequestration (CCS) in the United States; Congressional Research Service: Washington, DC, USA, 2021; Available online: https://sgp.fas.org/crs/misc/R44902.pdf (accessed on 29 November 2021).
- Sahin, S.; Kalfa, U.; Celebioglu, D. Bati Raman Field Immiscible CO2 Application--Status Quo and Future Plans. SPE Reserv. Eval. Eng. 2008, 11, 778–791. [Google Scholar] [CrossRef]
- Wang, Z.M.; Song, G.-L.; Zhang, J. Corrosion Control in CO2 Enhanced Oil Recovery From a Perspective of Multiphase Fluids. Front. Mater. 2019, 6, 272. [Google Scholar] [CrossRef]
- Al-Bayati, D.; Saeedi, A.; Myers, M.; White, C.; Xie, Q. Supercritical CO2 flooding into sandstone reservoirs: Implication of miscible and immiscible displacement. In Proceedings of the One Curtin International Postgraduate Conference (OCPC 2017), Sarawak, Malaysia, 10–12 December 2017. [Google Scholar]
- Wang, S.; Han, J.; Wang, Y.; Ling, K.; Jia, B.; Wang, H.; Long, Y. Recovery Potential and Mechanism Investigation of the Supercritical CO2 EOR in the Bakken Tight Formation. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 15–18 June 2019. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.; Liu, Y.; Jiang, L.; Zhu, N. Visualization of CO2 and oil immiscible and miscible flow processes in porous media using NMR micro-imaging. Pet. Sci. 2011, 8, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Zekri, A.Y.; Almehaideb, R.A.; Shedid, S.A. Displacement Efficiency of Supercritical CO2 Flooding in Tight Carbonate Rocks Under Immiscible Conditions. In Proceedings of the SPE Europec/EAGE Annual Conference and Exhibition, Vienna, Austria, 12–15 June 2006. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Fu, W.; Lu, X.; Chen, Z.; Qi, S.; Wang, Y.; Yang, W.; Lu, J. Extraction of shale oil with supercritical CO2: Effects of number of fractures and injection pressure. Fuel 2021, 285, 118977. [Google Scholar] [CrossRef]
- Irawan, F.; Irawan, S.; Awang, M. A Unique Opportunity for Liquid Carbon Dioxide as an Enhanced Oil Recovery Method. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 654–661. [Google Scholar] [CrossRef]
- Valluri, M.K.; Zhou, J.; Mishra, S.; Mohanty, K. CO2 Injection and Enhanced Oil Recovery in Ohio Oil Reservoirs—An Experimental Approach to Process Understanding. Energies 2020, 13, 6215. [Google Scholar] [CrossRef]
- Fahandezhsaadi, M.; Amooie, M.A.; Hemmati-Sarapardeh, A.; Ayatollahi, S.; Schaffie, M.; Ranjbar, M. Laboratory evaluation of nitrogen injection for enhanced oil recovery: Effects of pressure and induced fractures. Fuel 2019, 253, 607–614. [Google Scholar] [CrossRef]
- Tu, H.; Guo, P.; Jia, N.; Zhou, D.; Zhou, X.; Wang, Z. Determination of the Diffusion Coefficient of Supercritical CO2 in Low-Permeability Formation Cores. Energy Fuels 2020, 34, 2001–2014. [Google Scholar] [CrossRef]
- Voisin, T.; Erriguible, A.; Ballenghien, D.; Mateos, D.; Kunegel, A.; Cansell, F.; Aymonier, C. Solubility of inorganic salts in sub- and supercritical hydrothermal environment: Application to SCWO processes. J. Supercrit. Fluids 2017, 120, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, S. Supercritical Water Oxidation for Environmentally Friendly Treatment of Organic Wastes. Adv. Supercrit. Fluids Technol. 2020, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Hui, K.; Tang, J.; Lu, H.; Xi, B.; Qu, C.; Li, J. Status and prospect of oil recovery from oily sludge:A review. Arab. J. Chem. 2020, 13, 6523–6543. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Li, D.; Chen, H.; Xu, Y. Continuous supercritical water oxidation treatment of oil-based drill cuttings using municipal sewage sludge as diluent. J. Hazard. Mater. 2020, 384, 121225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.; Yin, F.; Wang, G.; Chen, H.; He, C.; Xu, Y. Supercritical water oxidation of oil-based drill cuttings. J. Hazard. Mater. 2017, 332, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, R.; Ni, H.; Wang, K. Experimental study on harmless disposal of waste oil based mud using supercritical carbon dioxide extraction. Fuel 2019, 252, 722–729. [Google Scholar] [CrossRef]
- Khairutdinov, V.F.; Akhmetzyanov, T.R.; Gabitov, F.R.; Zaripov, Z.I.; Farakhov, M.I.; Mukhutdinov, A.V.; Gumerov, F.M. Disposal of oil sludge with the use of liquid and supercritical fluid extraction processes with propane-butane extractant. Contemp. Eng. Sci. 2016, 9, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; He, H.; Zhang, Y. Optimization of oil based drilling cuttings treatment process by su-percritical CO2 fluid using response surface methodology. Chin. J. Environ. Eng. 2017, 11, 6050–6055. [Google Scholar] [CrossRef]
- Khayrutdinov, V.F.; Gabitova, A.R.; Gumerov, F.M.; Gabitov, R.F.; Kurdyukov, A.I. Extraction of Petroleum Products and Asphaltene–Resin Mixtures from Highly Watered Oil Sludge with Supercritical Fluid Method. Russ. J. Phys. Chem. B 2019, 13, 1128–1130. [Google Scholar] [CrossRef]
- Gumerov, F.M.; Khairutdinov, V.F.; Farakhov, M.I. Mathematical modeling of technology for disposal of oil sludge with the use of liquid and supercritical fluid extraction processes. J. Eng. Appl. Sci. 2016, 11, 2024–2027. [Google Scholar] [CrossRef]
- Ishida, T.; Chen, Y.; Bennour, Z.; Yamashita, H.; Inui, S.; Nagaya, Y.; Naoi, M.; Chen, Q.; Nakayama, Y.; Nagano, Y. Features of CO2 fracturing deduced from acoustic emission and microscopy in laboratory experiments. J. Geophys. Res. Solid Earth 2016, 121, 8080–8098. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Aoyagi, K.; Niwa, T.; Chen, Y.; Murata, S.; Chen, Q.; Nakayama, Y. Acoustic emission monitoring of hydraulic fracturing laboratory experiment with supercritical and liquid CO2. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhu, W.; Xu, Z.; Liu, S.; Wei, C. A review of experimental apparatus for supercritical CO2 fracturing of shale. J. Pet. Sci. Eng. 2022, 208, 109515. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Zhang, X.; Liu, G.; Du, X.; Shang, D.; Yu, Y.; Chen, J.; Wang, H.; Tu, H. Supercritical CO2 fracturing with different drilling depths in shale. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–20. [Google Scholar] [CrossRef]
- Wang, K. High-Pressure Study of Weak Intermolecular Interactions in Micelles; Jilin University: Jilin, China, 2005. [Google Scholar]
- Mazza, R.L. Liquid-Free CO2/Sand Stimulations: An Overlooked Technology-Production Update. In Proceedings of the SPE Eastern Regional Meeting, Canton, OH, USA, 17–19 October 2001. SPE-72383-MS. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, C.; Kang, Z.; Zhou, J.; Li, Y.; Liu, H.; Song, X. Experimental study of supercritical CO2 fracturing on initiation pressure and fracture propagation in shale under different triaxial stress conditions. J. Nat. Gas Sci. Eng. 2018, 55, 382–394. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, N.; Xian, X.; Zhou, L.; Tang, J.; Kang, Y.; Wang, H. Supercritical CO2 fracking for enhanced shale gas recovery and CO2 sequestration: Results, status and future challenges. Adv. Geo-Energy Res. 2019, 3, 207–224. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, Y.; Yin, C.; Li, X. Hydraulic Fracturing Behavior in Shale with Water and Supercritical CO2 under Triaxial Compression. Geofluids 2020, 2020, 4918087. [Google Scholar] [CrossRef] [Green Version]
- Sharifullin, A.V.; Baibekova, L.R.; Suleymanov, A.T. Features of the composition and structure of oil deposits. Oil Gas Technol. 2006, 6, 19–24. [Google Scholar]
- Sharifullin, A.V.; Baibekova, L.R.; Khamidullin, R.F. Composition and structure of asphalt-resin-paraffin deposits of the Tatarstan. Tekhnologii Nefti I Gaza 2006, 4, 34–41. [Google Scholar]
- Zheng, F.; Shi, Q.; Vallverdu, G.S.; Giusti, P.; Bouyssiere, B. Fractionation and Characterization of Petroleum Asphaltene: Focus on Metalopetroleomics. Processes 2020, 8, 1504. [Google Scholar] [CrossRef]
- Guiliano, M.; Boukir, A.; Doumenq, P.; Mille, G.; Crampon, C.; Badens, E.; Charbit, G. Supercritical Fluid Extraction of Bal 150 Crude Oil Asphaltenes. Energy Fuels 2000, 14, 89–94. [Google Scholar] [CrossRef]
- Magomedov, R.N.; Pripakhaylo, A.; Foteeva, L.S.; Maryutina, T.A. Method for Isolating Asphaltenes from Petroleum by Their Precipitation from Supercritical Carbon Dioxide. Chem. Technol. Fuels Oils 2019, 55, 287–298. [Google Scholar] [CrossRef]
- Sun, G.; Li, C.; Yang, S.; Yang, F.; Chen, Y. Experimental Investigation of the Rheological Properties of a Typical Waxy Crude Oil Treated with Supercritical CO2 and the Stability Change in Its Emulsion. Energy Fuels 2019, 33, 4731–4739. [Google Scholar] [CrossRef]
- Kozhevnikov, I.; Nuzhdin, A.; Martyanov, O. Transformation of petroleum asphaltenes in supercritical water. J. Supercrit. Fluids 2010, 55, 217–222. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Soltani, M.; Noofeli, A.; Nathwani, J. An optimization study on heavy oil upgrading in supercritical water through the response surface methodology (RSM). Fuel 2020, 271, 117618. [Google Scholar] [CrossRef]
- Shoji, E.; Kikuchi, T.; Yamagiwa, K.; Kubo, M.; Tsukada, T.; Takami, S.; Sugimoto, K.; Ito, D.; Saito, Y. In-situ visualization of heavy oil behavior in supercritical water using neutron radiography. Chem. Eng. Sci. 2020, 225, 115816. [Google Scholar] [CrossRef]
- Moiseeva, E.G.; Lakhova, A.I.; Petrov, S.M.; Bashkirtseva, N.Y. Conversion of High-Viscosity Oil of Ashalchinsk Deposit in the Presence of Activated Carbon and Supercritical Aqueous Fluid. Chem. Technol. Fuels Oils 2021, 57, 746–752. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Fedyaeva, O.N.; Grinko, A.A.; Vostrikov, A.A. Structural parameters of resins and asphaltenes of natural asphaltite and products of its conversion in supercritical water. AIP Conf. Proc. 2020, 2310, 020022. [Google Scholar] [CrossRef]
- Fakher, S.; Ahdaya, M.; Elturki, M.; Imqam, A. Critical review of asphaltene properties and factors impacting its stability in crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, J.; Qi, C.; Li, X.; Mai, T.; Zhang, J. Mechanism of asphaltene aggregation induced by supercritical CO2: Insights from molecular dynamics simulation. RSC Adv. 2017, 7, 50786–50793. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-S.; Srdjan, N. Corrosion Behavior Of Carbon Steel in Supercritical CO2–Water Environments. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 12–16 March 2009. NACE-09256. [Google Scholar]
- Paschke, B.; Kather, A. Corrosion of Pipeline and Compressor Materials Due to Impurities in Separated CO2 from Fossil-Fuelled Power Plants. Energy Procedia 2012, 23, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.E.; Meyer, J.P.; Meadows, S.R. Carbon Dioxide Enhanced Oil Recovery Injection Operations Technologies (Poster Presentation). Energy Procedia 2009, 1, 3141–3148. [Google Scholar] [CrossRef] [Green Version]
- de Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control. 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Oosterkamp, A.; Ramsen, J. State-of-the-Art Overview of CO2 Pipeline Transport with Relevance to Offshore Pipelines; Polytec: Haugesund, Norway, 2008. [Google Scholar]
- Wei, L.; Zhang, Y.; Pang, X.; Gao, K. Corrosion behaviors of steels under supercritical CO2 conditions. Corros. Rev. 2015, 33, 151–174. [Google Scholar] [CrossRef]
- Marrone, P.A.; Hong, G.T. Corrosion control methods in supercritical water oxidation and gasification processes. J. Supercrit. Fluids 2009, 51, 83–103. [Google Scholar] [CrossRef]
- Mahlobo, M.; Premlall, K.; Olubambi, P. Effect of CO2 partial pressure and different CO2 phases on carbon steel corrosion. IOP Conf. Series Mater. Sci. Eng. 2017, 272, 012032. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.; Choi, Y.-S. State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: Mechanisms and models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Dugstad, A.; Halseid, M.; Morland, B.H. Effect of SO2 and NO2 on Corrosion and Solid Formation in Dense Phase CO2 Pipelines. Energy Procedia 2013, 37, 2877–2887. [Google Scholar] [CrossRef] [Green Version]
- Teeter, L.; Adam, B.; Wood, T.; Tucker, J. Comparison of the corrosion of materials in supercritical carbon dioxide, air, and argon environments. Corros. Sci. 2021, 192, 109752. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Gong, B.; Jiang, E.; Huang, Y.; Zhang, G.; Zhao, Y. Corrosion behavior of typical structural steels in 500 °C, 600 °C and high pressure supercritical carbon dioxide conditions. Corros. Sci. 2021, 192, 109801. [Google Scholar] [CrossRef]
- Bidabadi, M.H.S.; Zhang, C.; Chen, H.; Yang, Z.-G. Temperature dependence of carbon deposits within oxide scale on CrMoV steel in atmospheric and supercritical CO2. Corros. Sci. 2022, 195, 109979. [Google Scholar] [CrossRef]
- Yin, Z.F.; Feng, Y.R.; Zhao, W.Z.; Bai, Z.Q.; Lin, G.F. Effect of temperature on CO2corrosion of carbon steel. Surf. Interface Anal. 2009, 41, 517–523. [Google Scholar] [CrossRef]
- Schmitt, G.; Horstemeier, M. Fundamental Aspects of CO2 Metal Loss Corrosion-Part II: Influence of Dif-ferent Parameters on CO2 Corrosion Mechanisms. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- Lin, G.; Zheng, M.; Bai, Z.; Zhao, X. Effect of Temperature and Pressure on the Morphology of Carbon Dioxide Corrosion Scales. Corrosion 2006, 62, 501–507. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, C.; Cui, G.; Xing, X.; Mu, J.; Li, Z. Analysis of internal corrosion of supercritical CO2 pipeline. Corros. Rev. 2021, 39, 219–241. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, C.; Hesitao, W.; Long, Z.; Yan, W. Understanding the pitting corrosion mechanism of pipeline steel in an impure supercritical CO2 environment. J. Supercrit. Fluids 2018, 138, 132–142. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Z.; Harris, M.A.; Sánchez, L.J.; Cong, H. CO2 Corrosion of Low Carbon Steel Under the Joint Effects of Time-Temperature-Salt Concentration. Front. Mater. 2019, 6, 10. [Google Scholar] [CrossRef]
- Duthie, L.; Almarri, H.; Saiood, H.; Lata, A.; Gokul, R. Effective Corrosion Mitigation Exploiting Glass Reinforced Epoxy Lined Tubulars in Offshore Producing & Injection Wells. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2019. [Google Scholar] [CrossRef]



| Solvent | Critical Parameters | ||
|---|---|---|---|
| Temperature, °C | Pressure, MPa | Density, kg/m3 | |
| Carbon dioxide | 31.3 | 7.29 | 468 |
| Ammonia | 123.3 | 11.13 | 235 |
| Water | 374.4 | 22.65 | 322 |
| Methanol | 240.5 | 7.89 | 278 |
| Ethanol | 243.4 | 6.3 | 276 |
| Isopropanol | 235.2 | 4.7 | 274 |
| Ethan | 32.4 | 4.83 | 203 |
| Propane | 96.8 | 4.2 | 217 |
| n-Butane | 152 | 3.75 | 225 |
| n-Pentane | 196.6 | 3.33 | 232 |
| n-Hexane | 234.2 | 2.96 | 234 |
| Benzene | 288.9 | 4.83 | 302 |
| Chlorotrifluoromethane | 28.8 | 3.9 | 579 |
| Nitric oxide | 36.5 | 7.14 | 450 |
| Component | Concentration | Limitation |
|---|---|---|
| H2O | 500 ppm | Technical: below solubility limit of H2O in CO2, no significant cross effect of H2O and H2S, cross effect of H2O and CH4 is significant but within limit for water solubility |
| H2S | 200 ppm | Health and Safety consideration |
| CO | 200 ppm | Health and Safety consideration |
| O2 | Aquifer < 4 vol%. EOR 100–1000 ppm | Technical: range for EOR, because of lack of practical experiments on the effects of O2 underground |
| CH4 | Aquifer < 4 vol%. EOR <2 vol% | Health and Safety consideration |
| N2 | <4 vol% (all noncondensable gases) | As proposed in ENCAP project |
| Ar | <4 vol% (all noncondensable gases) | As proposed in ENCAP project |
| H2 | <4 vol% (all noncondensable gases) | Further reduction of H2 is recommended because of its energy content |
| SOx | 100 ppm | Health and Safety consideration |
| NOx | 100 ppm | Health and Safety consideration |
| CO2 | >95.5% | Balance with other compounds in CO2 |
| Equipment, Constructions | Materials |
|---|---|
| Measuring sections of the pipeline | 316 SS, fiberglass |
| Christmas tree (Trim) | 316 SS, nickel, monel-metal |
| Valve packing and seals | Teflon, nylon |
| Wellhead (Trim) | 316 SS, nickel, monel-metal |
| Tubing Hanger | 316 SS, Incoloy |
| Tubing String | Glass Reinforced Epoxy (GRE), Internal Plastic Coating (IPC), Corrosion-Resistant Alloy (CRA) |
| Tubing Joint Seals | Seal ring (GRE), Coated threads and collars (IPC) |
| ON/OFF Tool, Profile Nipple | Nickel plated wetted parts, 316 SS |
| Packers | Internally coated hardened rubber of 80–90 durometer strength (Buna–N), Nickel plated wetted parts |
| Cements and cement additives | API cements and/or acid resistant specialty cements and additives |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, P.L.; Minakov, A.V.; Platonov, D.V.; Zhigarev, V.A.; Guzei, D.V. Supercritical Fluid Application in the Oil and Gas Industry: A Comprehensive Review. Sustainability 2022, 14, 698. https://doi.org/10.3390/su14020698
Pavlova PL, Minakov AV, Platonov DV, Zhigarev VA, Guzei DV. Supercritical Fluid Application in the Oil and Gas Industry: A Comprehensive Review. Sustainability. 2022; 14(2):698. https://doi.org/10.3390/su14020698
Chicago/Turabian StylePavlova, Praskovya L., Andrey V. Minakov, Dmitriy V. Platonov, Vladimir A. Zhigarev, and Dmitriy V. Guzei. 2022. "Supercritical Fluid Application in the Oil and Gas Industry: A Comprehensive Review" Sustainability 14, no. 2: 698. https://doi.org/10.3390/su14020698






